An improved understanding of the genome of Sarcocystis neurona, the primary cause of EPM
Our lab is working to solve equine protozoal myeloencephalitis (EPM), one of the most commonly diagnosed neurologic diseases in horses in North and South America. Annual direct costs associated with the diagnosis and treatment of EPM represents a large economic burden to the equine industry and is estimated to be between $55.4 million and $110.8 million in the United States alone. This does not account for the additional economic impact due to lost production, decreased performance and the cost of care during recovery. Even after successful veterinary intervention, full recovery from the disease can be difficult due to lasting damage to tissues of the central nervous system.
EPM is caused primarily by Sarcocystis neurona, a single-celled parasite. Normally, Sarcocystis has a two-host lifecycle where it spends most of its time in small mammals such as skunks, racoons and nine-banded armadillos. After asexual replication, parasites will form dormant cysts (sarcocysts) in the muscle tissue of this animal, which are later ingested through scavenging by the opossum definitive host. In the opossum, the parasites excyst in the intestines, where they sexually replicate to produce environmentally-stable infective sporocysts that are passed along with feces into the environment. Interestingly, the natural lifecycle of S. neurona does not include the horse. Horses become accidental hosts when they ingest feed or water contaminated with opossum feces containing these sporocysts. In the horse, the parasites excyst in the gut and migrate to the brain and/or spinal cord where they cause local inflammation and tissue damage. Depending on the location of this inflammation, clinical signs can vary to include slight incoordination, asymmetric muscle atrophy, partial facial paralysis, difficulty swallowing, ataxia, recumbence, seizures and/or death.
Of 5,250 horses sampled in 2013 from 18 states across the United States, seroprevalence of antibodies against S. neurona was 78% indicating that horses are commonly infected by this parasite. A study by the National Animal Health Monitoring System in 2001 reported the annual occurrence of EPM to be approximately 0.14% in the US, with case fatality rate at 4.7%. The large discrepancy between the seroprevalence of antibodies to S. neurona and the low occurrence of EPM is puzzling and suggests that disease pathogenesis is complex.
Initially revolutionized by the sequencing of the human genome, contemporary research now permits efficient investigation of the proteome, transcriptome, microbiome, etc. of any organism for which there is a high-quality genome sequence and annotation (identification of the structure and functional importance of individual genes). Using the horse as an example, the equine reference genome has allowed for identification of genetic variants associated with coat-color, dwarfism, cytokine expression and multiple other traits. Furthermore, the equine reference genome has made possible the Functional Annotation of Animal Genome (FAANG) project, a collaborative initiative promising to correlate and make available a tissue-specific transcriptome (identification of all the genes expressed in a specific cell type) and regulome (all of the regulatory components of a specific cell type) of the horse.
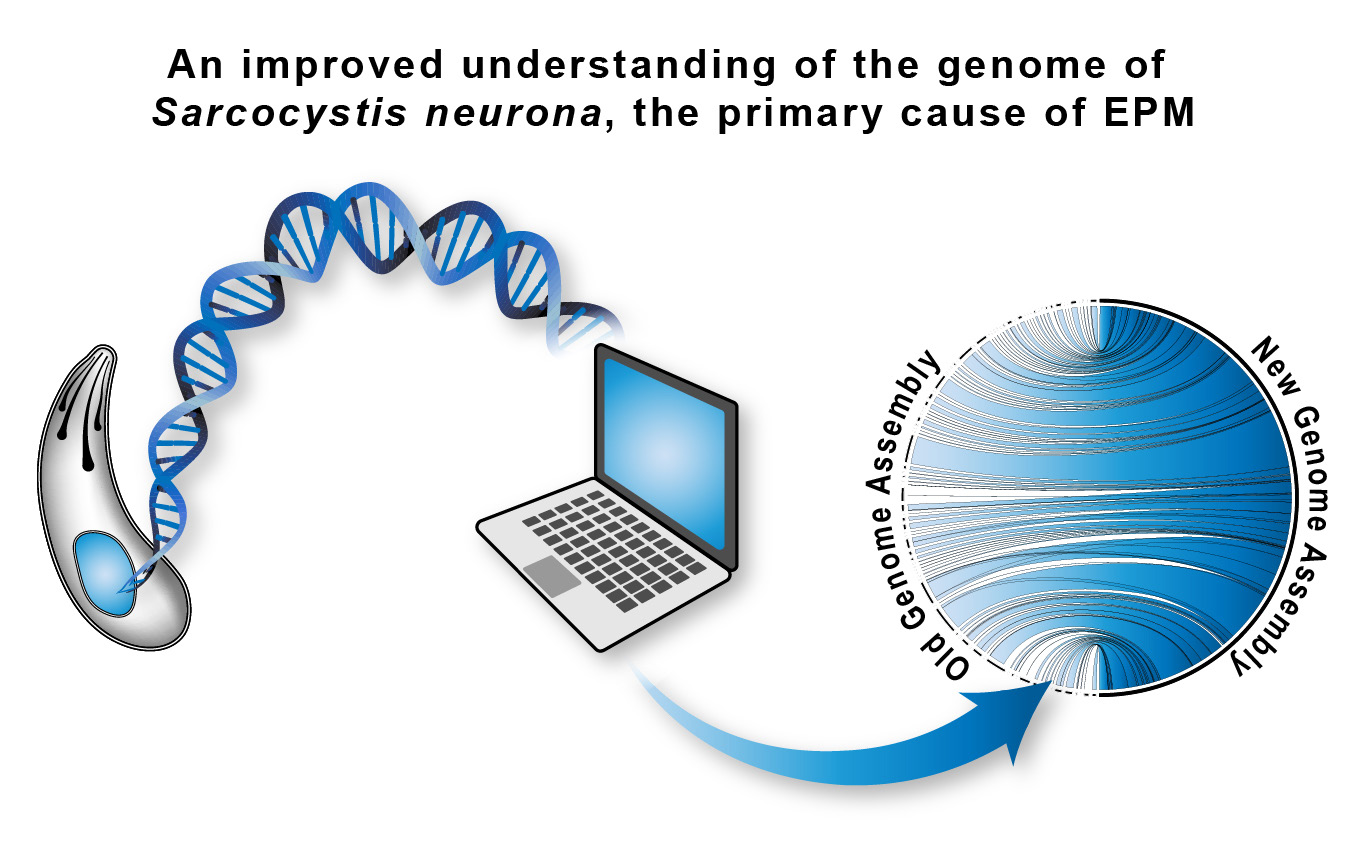
Like “omics” investigations in humans and horses, computational approaches represent a powerful toolset to improve our understanding of S. neurona and EPM. The current reference genome for S. neurona was produced more than a decade ago. Using state-of-the-art, next-generation sequencing technologies and improved computational methodologies, we have generated an updated reference genome for S. neurona that provides an improved assembly and more accurate genome annotation. Importantly, previous gene annotation for the S. neurona genome relied heavily on evidence from the “close” relatives Toxoplasma gondii and Neospora caninum. In this new genome, the structure of each gene has been painstakingly curated using evidence derived specifically from S. neurona, which should ensure the peculiarities caused by 250 million years of Sarcocystis evolution remain evident.
This collaboration between the University of Kentucky’s Gluck Equine Research Center, Purdue University and the University of Georgia will give us and other researchers a better understanding of the genetic “language” of S. neurona, which should lead to improved diagnostic methodologies, preventative measures and treatments for EPM.
Source: January 2024 Equine Disease Quarterly. Jamie Kaj Norris is a PhD Student in the Gluck Equine Research Center under researcher Dan Howe.
